
Company News
 2025-05-15
2025-05-15 90
90On May 15, 2025, ImmuneOnco Biopharmaceuticals (Shanghai) Inc. (referred to as “ImmuneOnco,” Hong Kong Stock Exchange stock code: 01541.HK) announced that the Phase Ib/II clinical trial application for tazlestobart (IMM27M) combined with osimertinib for the treatment of EGFR-mutated locally advanced or metastatic NsqNSCLC, has been officially approved by NMPA. This milestone marks another critical step in ImmuneOnco’s rapid development of tazlestobart (IMM27M) in clinical research.

EGFR-mutated locally advanced or metastatic NsqNSCLC is a common subtype of lung cancer, particularly in Asian populations where EGFR mutations are highly prevalent. While epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) (e.g., osimertinib) serves as the first-line standard treatment, drug resistance remains a significant challenge.
Studies have demonstrated that EGFR-TKI (e.g., osimertinib) induces upregulation of the immune checkpoint cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), thereby promoting immunosuppression in the tumor microenvironment. Combining CTLA-4 inhibitors with EGFR-TKI can restore T-cell effector function, reverse CTLA-4-mediated suppression, and enhance efficacy against TKI-resistant tumors.【1】
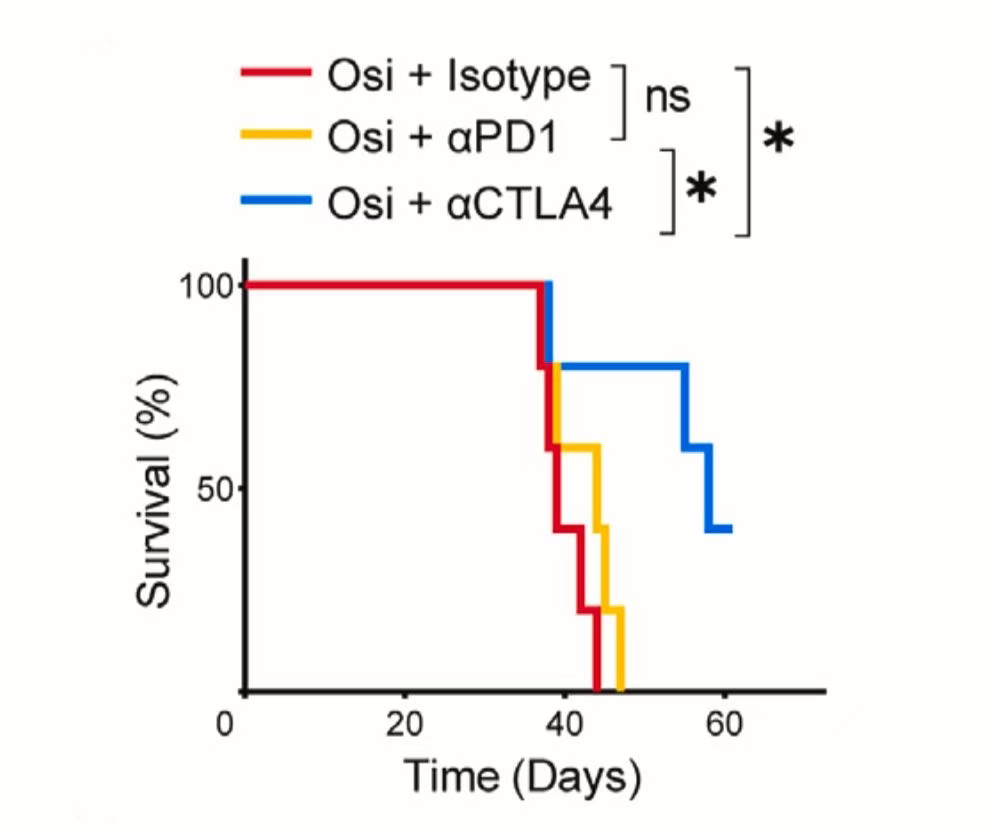
In mouse models, the combination of osimertinib with a CTLA-4 inhibitor significantly prolonged overall survival, demonstrating clear superiority over osimertinib monotherapy and osimertinib combined with a PD-1 antibody.
Tazlestobart (IMM27M), an Immunoglobulin G1 (IgG1) antibody targeting CTLA-4, has been genetically engineered to significantly enhance antibody-dependent cell-mediated cytotoxicity (ADCC) activity. It effectively depletes regulatory T cells in the tumor microenvironment, amplifying anti-tumor immune responses. This mechanism, when combined with EGFR-TKIs, may improve outcomes in drug-resistant patients.
Additionally, repeated in vivo studies have confirmed tazlestobart (IMM27M)’s robust anti-tumor activity and its potential for combination with a variety of other pipeline drugs. A clinical study of palverafusp alfa (IMM2510) combined with tazlestobart (IMM27M) in advanced solid tumors is currently enrolling patients.
Dr. Tian, Wenzhi, Founder, Chairman, CEO, and CSO of ImmuneOnco, said:
“The approval of this clinical trial for tazlestobart (IMM27M) combined with osimertinib represents another important advancement for ImmuneOnco in tumor immunotherapy. This approved clinical trial focuses on EGFR-mutated locally advanced or metastatic NsqNSCLC patients, whose unmet medical needs remain significant. If the combination demonstrates favorable safety and efficacy in clinical trials, it may offer a novel therapeutic option for this patient population. This achievement not only underscores ImmuneOnco’s innovation in drug development but also brings new hope and a brighter future to patients. We will go all out to advance the clinical trial process to ensure this innovative therapy benefits patients as soon as possible.”
Reference:【1】Minjie Fu, Jiaxu Zhao, Licheng Zhang, et al. Cancer Cell. 2024 Nov 11;42(11):1882-1897.e7.




For more information
Please follow the official wechat public account