Scientific Technology and R&D
IMM27M (CTLA-4 ADCC-enhanced mAb)
IMM27M is a new generation CTLA-4 antibody with enhanced ADCC activity through genetic engineering modification. As a protein receptor that can be found on the activated T cells, CTLA-4 can downregulate immune responses by binding to CD80/CD86, its natural ligands found on the surface of antigen presenting cells, delivering inhibitory signal and thus suppressing T-cell immune function. CTLA-4 antibodies can block the interaction between CTLA-4 and CD80/CD86, and thus enhance immune responses of T cells to tumor antigens.
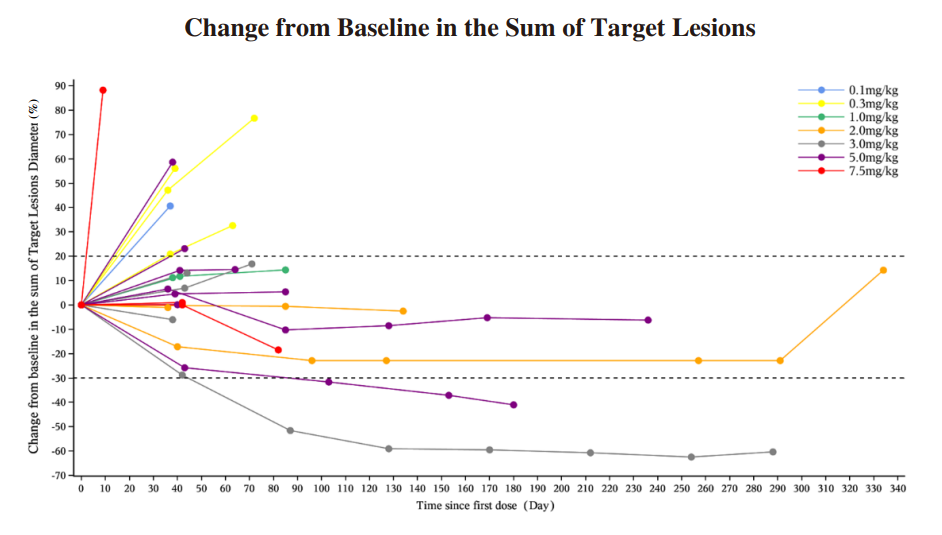
We have completed the enrollment of patients for the Phase I dose-escalation study of IMM27M in September 2023, and the preliminary data has demonstrated that IMM27M is safe and well tolerated. There was no DLT observed. The RP2D has been determined. In the Phase I dose-escalation study, we have observed 2 confirmed PRs, by June 30, 2024. We have also observed 3 SDs with tumor shrinkage. The following diagram illustrates the interim efficacy data of the IMM27M: