Scientific Technology and R&D
IMM2510 (VEGF×PD-L1)
IMM2510 is a bispecific molecule with the mAb-Trap structure that targets VEGF and PD-L1 for the treatment of solid tumors. By targeting VEGF and PD-L1, IMM2510 is able to activate T-cell tumor killing activities and simultaneously inhibit tumor angiogenesis and tumor growth. Moreover, IMM2510 can also activate NK cells and macrophages through Fc-mediated ADCC/ADCP activities.
o Monotherapy
◆ We completed the enrollment of patients for the Phase I dose-escalation study of IMM2510 in September 2023. Total 33 patients with advanced/metastatic solid tumors were enrolled and dosed. There was no DLT observed. The RP2D has been determined. The clinical data as of June 30, 2024 from the Phase I trial of IMM2510 has demonstrated tolerable safety and promising antitumor activity. As of June 30, 2024, we have observed three patients who confirmed PR. We observed seven patients with SD and four of them had over 15% tumor shrinkage. The following diagrams illustrate the interim efficacy data of IMM2510 monotherapy:
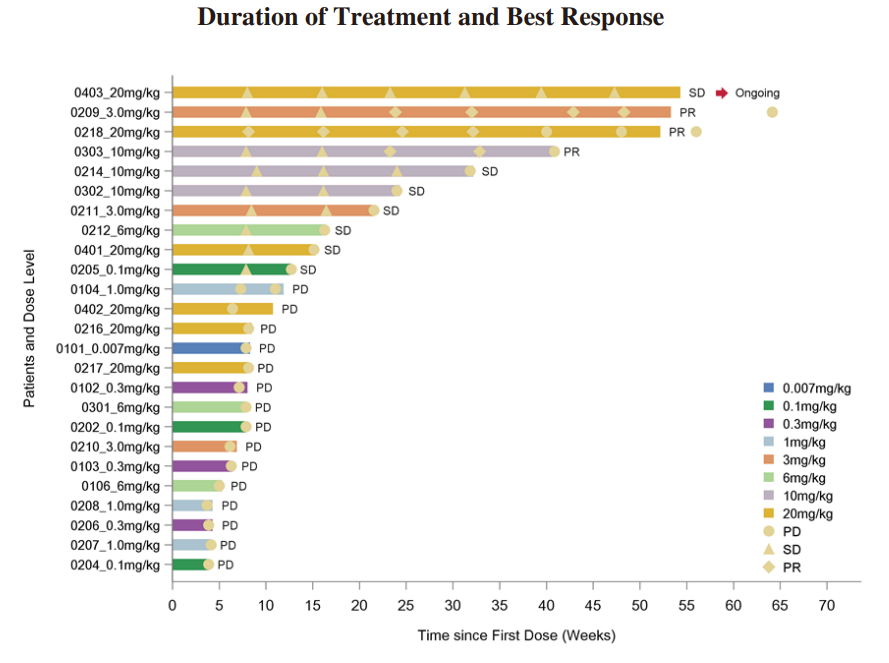
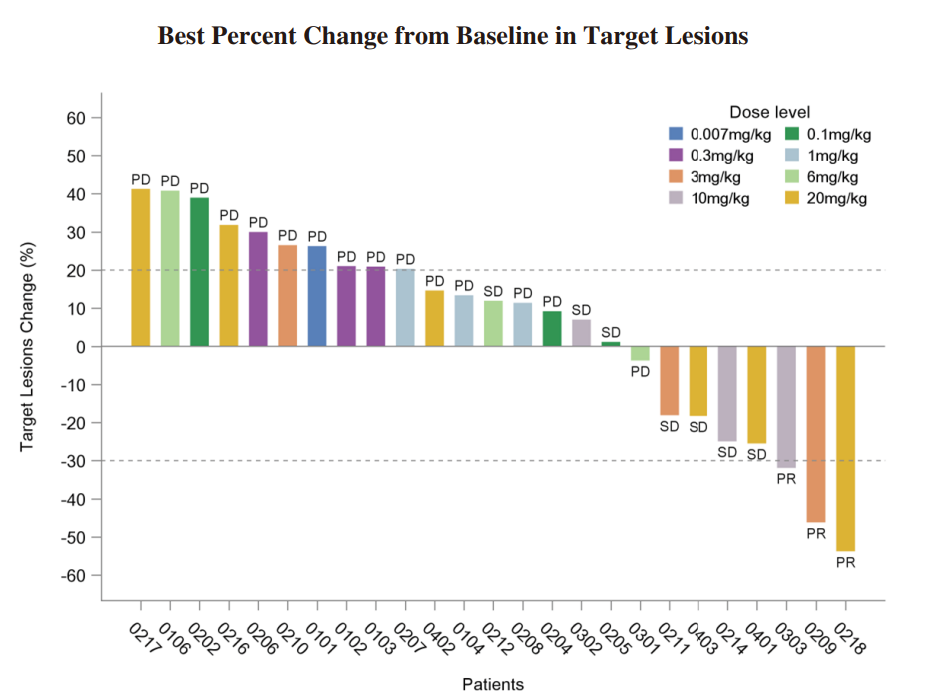
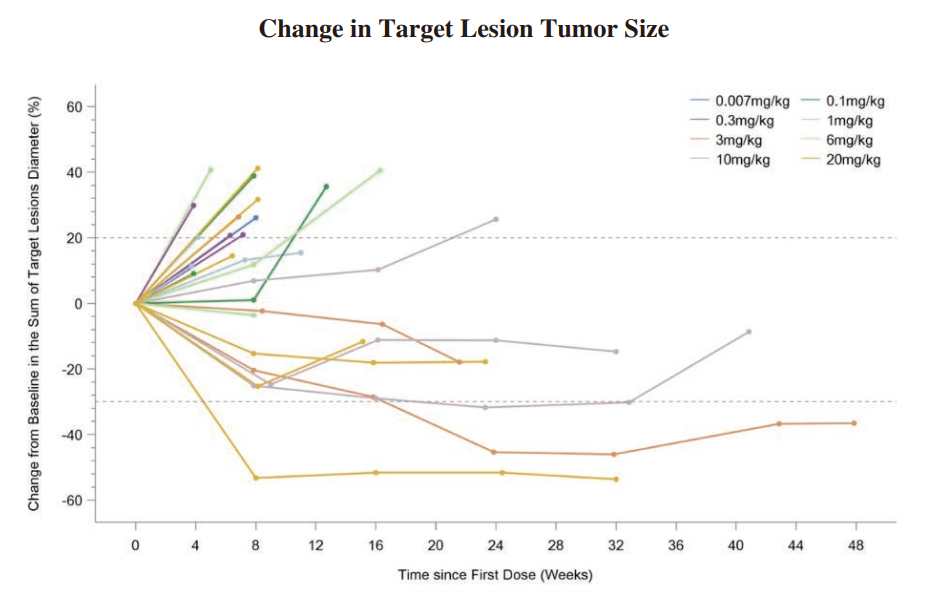
◆ We dosed the first patient in the Phase Ib/II clinical trial of IMM2510 in China in November 2023. As of June 30, 2024, three solid tumor patients were assessed PR by local investigator.