Scientific Technology and R&D
IMM01 (SIRPα-Fc Fusion Protein)
IMM01, our Core Product, is an innovative CD47-targeted molecule. It is the first SIRPα-Fc fusion protein to enter into clinical stage in China. IMM01 designed with IgG1 Fc can fully activate macrophages via a dual mechanism — simultaneously blocking the “don’t eat me” signal by disrupting CD47/ SIRPα interaction and delivering the “eat me” signal through the engagement of activating Fcγ receptors on macrophages. Furthermore, the CD47-binding
domain of IMM01 was specifically engineered to avoid human red blood cell (RBC) binding. With the differentiated molecule design, IMM01 has achieved a favorable safety profile and demonstrated its ability to activate macrophages. Moving forward, we may actively explore IMM01’s therapeutic potential in other indications and seek collaboration opportunities.
During the Reporting Period and up to the date of this announcement, we have achieved the following progress and milestones:
o Combination Therapy with Azacitidine
◆ The FDA has granted an orphan-drug designation to IMM01 in combination with azacitidine for the treatment of CMML in November 2023.
◆ We have completed the enrollment of patients for the Phase II clinical trial of IMM01 in combination with azacitidine for the first-line treatment of higher-risk MDS in June 2023. 57 patients were enrolled in the study. As of June 30, 2024, among the 51 efficacy evaluable patients, ORR was 64.7% (33/51), including 33.3% of patients (17/51) achieved CR, 15.7% of patients reached mCR with hematologic improvement (HI), 3.9% of patients reached HI and 11.8% of patients reached mCR alone. For patients treated for ≥ 4 months, the ORR reached 85.3% (29/34), and the CRR was 50.0% (17/34). Among patients treated for ≥ 6 months, the ORR reached 89.7% (26/29), and the CRR was 58.6% (17/29), demonstrating increasing efficacy with prolonged treatment duration. Without having to resort to priming dose, the Grade ≥3 hemolysis was rare (only 1.8%). IMM01 (without low-dose priming) combined with azacitidine were well tolerated and showed exciting efficacy results in patients with treatment-naïve higher-risk MDS, as demonstrated in the diagram below:
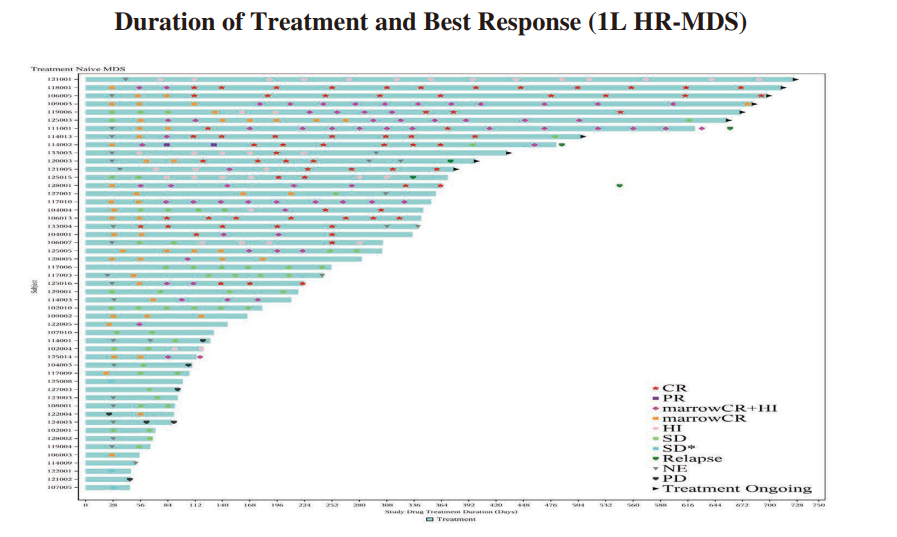

◆ A randomized, controlled, double-blind, multicenter, Phase III study (IMM01-009) of IMM01 (Timdarpacept) in combination with azacitidine in patients with newly diagnosed higher-risk MDS was approved by NMPA in May 2024.
◆ We completed the enrollment of patients for the Phase II clinical trial of IMM01 in combination with azacitidine for the first-line treatment of CMML in May 2023. 24 patients were enrolled. As of June 30, 2024, among the 22 efficacy evaluable patients, the ORR was 72.7% (16/22), including 27.3% of patients (6/22) achieved CR, 13.6% of patients reached marrow CR (mCR) with hematologic improvement (HI), 4.5% of patients reached HI and 27.3% of patients reached mCR alone. In patients treated for ≥ 4 months, the ORR reached 87.5%
(14/16), and the CRR was 37.5% (6/16). Among patients treated for ≥ 6 months, the ORR reached 84.6% (11/13), and the CRR was 46.2% (6/13), revealing increasing efficacy with prolonged treatment duration. IMM01, without the use of low-dose priming, combined with azacitidine, was well tolerated in 1L CMML. The combination of IMM01 with azacitidine, showed exciting efficacy results for patients with treatment-naive CMML, as demonstrated in the diagram below:
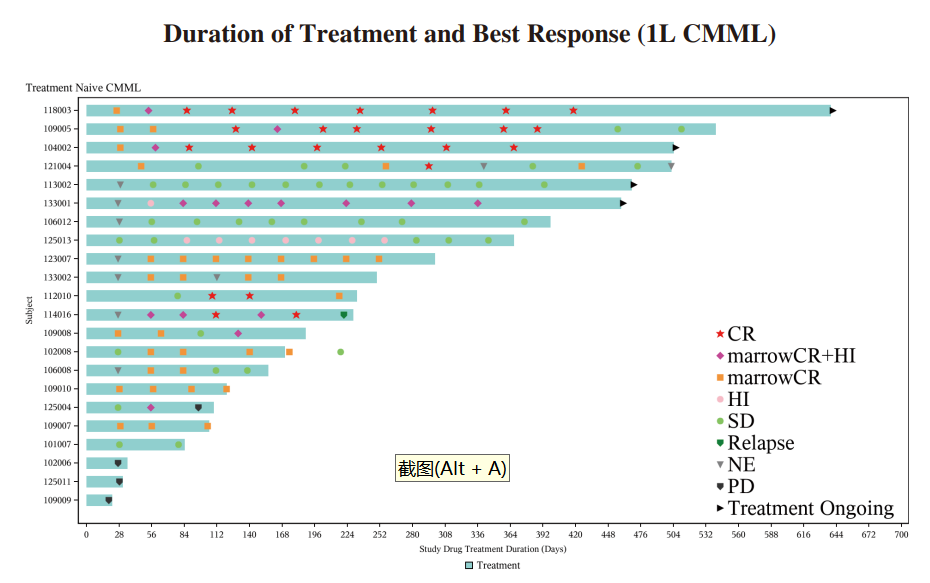
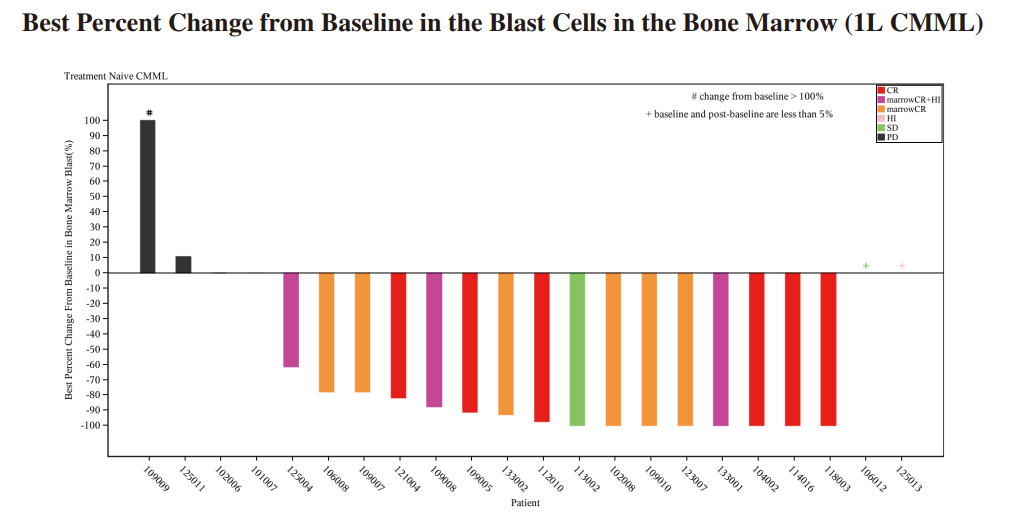
◆ A randomized, controlled, double-blind, multicenter, Phase III study (IMM01–010) of IMM01 (Timdarpacept) in combination with azacitidine in patients with newly diagnosed CMML was approved by NMPA in May 2024.