
Company News
 2024-11-13
2024-11-13 955
955The board (the “Board”) of directors (“Directors”, and each a “Director”) of the Company is pleased to announce that the Company has initiated the Phase II clinical trial of IMM27M for estrogen receptor positive (ER+) advanced breast cancer that failed after endocrine therapy or recurred and has enrolled the first patient. In addition, the Phase I dose-escalation study of IMM27M was completed in late 2023, demonstrating the following results (as of August 6, 2024): • In the Phase I trial, a total of eight evaluable ER+ advanced or metastatic breast cancer patients were enrolled. Among them, two achieved partial response (PR) and four patients had stable disease (SD), resulting in an overall response rate (ORR) of 25.0% and a disease control rate (DCR) of 75.0%; • Positive preliminary efficacy signals were demonstrated; and • IMM27M was found to be safe and well-tolerated, with no dose-limiting toxicity observed at the highest explored dose level of 7.5 mg/kg in Phase I.
The following diagrams illustrate the efficacy evaluation data of the IMM27M Phase I dose-escalation study:
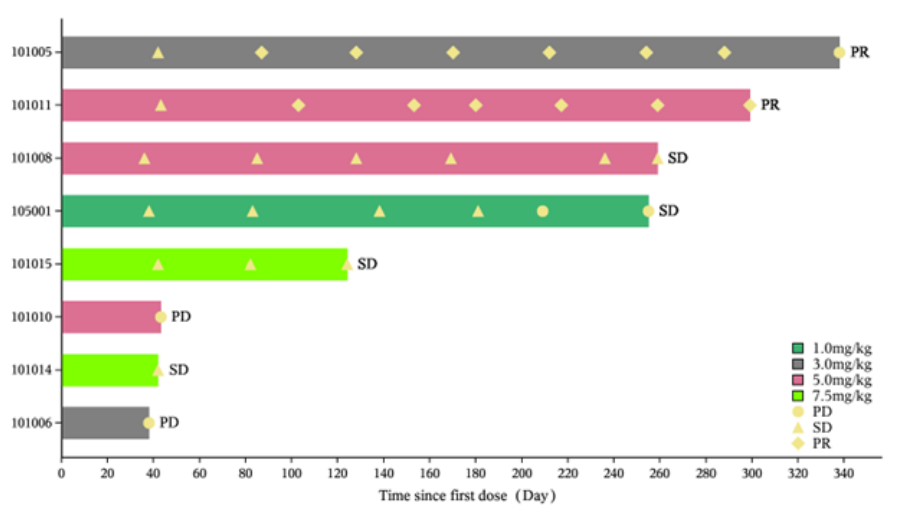 Abbreviations: PD refers to progressive disease; mBC refers to metastatic breast cancer; EAS refers to
endocrine active substances.
Abbreviations: PD refers to progressive disease; mBC refers to metastatic breast cancer; EAS refers to
endocrine active substances.
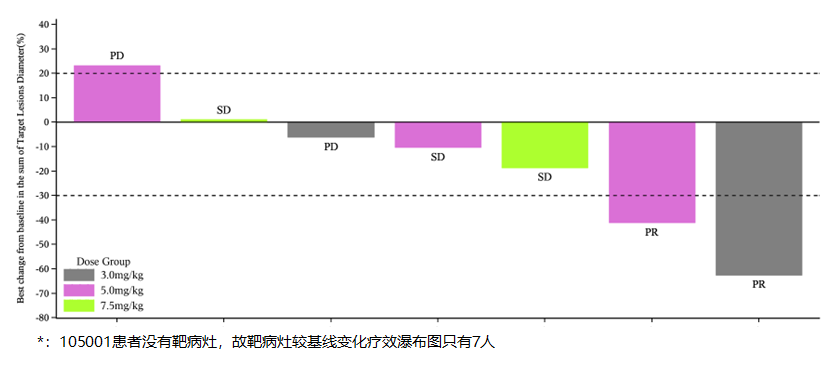 The recommended Phase II dose (RP2D) for monotherapy has been determined to be 5.0
mg/kg administered once every three weeks (Q3W).
The recommended Phase II dose (RP2D) for monotherapy has been determined to be 5.0
mg/kg administered once every three weeks (Q3W).
ABOUT IMM27MIMM27M is a new generation cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)antibody with enhanced antibody-dependent cellular cytotoxicity (ADCC) activity. It caninduce potent immune responses targeting CTLA-4 overexpressed immune-suppressive Tregulatory (Treg) cells and promote Treg depletion from the tumor microenvironment (TME),thus enhancing T-cell antitumor response.




For more information
Please follow the official wechat public account